2.3.1.82: aminoglycoside 6'-N-acetyltransferase
This is an abbreviated version!
For detailed information about aminoglycoside 6'-N-acetyltransferase, go to the full flat file.
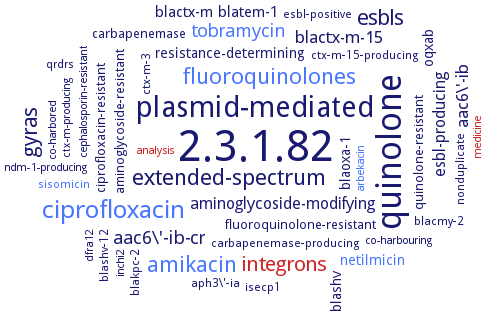
Word Map on EC 2.3.1.82 
-
2.3.1.82
-
quinolone
-
plasmid-mediated
-
ciprofloxacin
-
amikacin
-
extended-spectrum
-
fluoroquinolones
-
gyras
-
esbls
-
integrons
-
aac6\'-ib-cr
-
tobramycin
-
aac6\'-ib
-
blactx-m-15
-
esbl-producing
-
aminoglycoside-modifying
-
blatem-1
-
blactx-m
-
blashv
-
blaoxa-1
-
netilmicin
-
oqxab
-
resistance-determining
-
fluoroquinolone-resistant
-
ciprofloxacin-resistant
-
quinolone-resistant
-
carbapenemase
-
aminoglycoside-resistant
-
ctx-m-3
-
blacmy-2
-
isecp1
-
aph3\'-ia
-
qrdrs
-
blakpc-2
-
carbapenemase-producing
-
nonduplicate
-
sisomicin
-
blashv-12
-
ctx-m-15-producing
-
esbl-positive
-
co-harbored
-
dfra12
-
arbekacin
-
inchi2
-
co-harbouring
-
ctx-m-producing
-
analysis
-
ndm-1-producing
-
cephalosporin-resistant
-
medicine
- 2.3.1.82
-
quinolone
-
plasmid-mediated
- ciprofloxacin
- amikacin
-
extended-spectrum
- fluoroquinolones
-
gyras
-
esbls
- integrons
-
aac6\'-ib-cr
- tobramycin
-
aac6\'-ib
- blactx-m-15
-
esbl-producing
-
aminoglycoside-modifying
- blatem-1
-
blactx-m
-
blashv
- blaoxa-1
- netilmicin
-
oqxab
-
resistance-determining
-
fluoroquinolone-resistant
-
ciprofloxacin-resistant
-
quinolone-resistant
- carbapenemase
-
aminoglycoside-resistant
-
ctx-m-3
- blacmy-2
-
isecp1
-
aph3\'-ia
-
qrdrs
- blakpc-2
-
carbapenemase-producing
-
nonduplicate
- sisomicin
-
blashv-12
-
ctx-m-15-producing
-
esbl-positive
-
co-harbored
-
dfra12
- arbekacin
-
inchi2
-
co-harbouring
-
ctx-m-producing
- analysis
-
ndm-1-producing
-
cephalosporin-resistant
- medicine
Reaction
Synonyms
6'-acetyltransferase-Im, 6'-N-acetyltransferase type Ib, AAC, aac(3)-Ib/aac(6')-Ib, AAC(3)-Ib/AAC(6')-Ib', AAC(6'), AAC(6')-I, AAC(6')-Iaf, AAC(6')-IaI, AAC(6')-Iaj, AAC(6')-Ian, AAC(6')-Ib, AAC(6')-Ib-cr, AAC(6')-Ie, AAC(6')-Ie/APH(2")-Ia, AAC(6')-Ie/APH(2'')-Ia, AAC(6')-Ie/APH(2')-Ia, AAC(6')-Ig, AAC(6')-Ih, AAC(6')-Ii, AAC(6')-Im, AAC(6')-Isa, AAC(6')-Iy, AAC(6')-Iz acetyltransferase, AAC(6)-Iak, aac(6)-Ib, AAC3-I, aacA-aphD, acetyltransferase, aminoglycoside 6'-N-, acetyltransferase, kanamycin, acetyltransferase-6'-aminoglycoside phosphotransferase-2", aminoglycoside (3) acetyltransferase AAC(3)-Ia, aminoglycoside 3-N-acetyltransferase/aminoglycoside 6'-N-acetyltransferase fusion protein, aminoglycoside 6'-acetyltransferase-Ie, aminoglycoside 6'-N-acetyltransferase, aminoglycoside 6'-N-acetyltransferase Ib, aminoglycoside 6'-N-acetyltransferase type Ib, aminoglycoside 6'-N-acetyltransferase type Ii, aminoglycoside 6-N-acetyltransferase, aminoglycoside acetyltransferase, aminoglycoside acetyltransferase eis, aminoglycoside acetyltransferase(6')-Ie/aminoglycoside phosphotransferase(2')-Ia, aminoglycoside N(6')-acetyltransferase type 1, aminoglycoside N-6'-acetyltransferase, aminoglycoside N-6'-acetyltransferase Ii, aminoglycoside N-acetyltransferase, aminoglycoside-6'-acetyltransferase, aminoglycoside-6'-N-acetyltransferase, aminoglycoside-6-N-acetyltransferase, ANT(3'')-Ii/AAC(6')-IId, EC 2.3.1.55, meta-AAC0020

 results (
results ( results (
results ( top
top






