1.16.98.B1: mitochondrial amidoxime reducing component
This is an abbreviated version!
For detailed information about mitochondrial amidoxime reducing component, go to the full flat file.
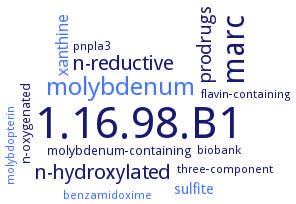
Word Map on EC 1.16.98.B1 
-
1.16.98.B1
-
marc
-
molybdenum
-
n-reductive
-
n-hydroxylated
-
prodrugs
-
xanthine
-
sulfite
-
molybdenum-containing
-
n-oxygenated
-
molybdopterin
-
biobank
-
three-component
-
pnpla3
-
benzamidoxime
-
flavin-containing
- 1.16.98.B1
-
marc
- molybdenum
-
n-reductive
-
n-hydroxylated
-
prodrugs
- xanthine
- sulfite
-
molybdenum-containing
-
n-oxygenated
- molybdopterin
-
biobank
-
three-component
-
pnpla3
- benzamidoxime
-
flavin-containing
Reaction
2 [Fe(II)-cytochrome b5]
+
2 H+
+
Synonyms
B4114_1419, mARC1, mARC2, mitochondrial amidoxime reducing component 2, mitochondrial amidoxime-reducing component 1, Moco sulfurase C-terminal domain protein, MOSC domain-containing protein, MTARC1, MTARC2, YiiM
ECTree
Advanced search results
Crystallization
Crystallization on EC 1.16.98.B1 - mitochondrial amidoxime reducing component
Please wait a moment until all data is loaded. This message will disappear when all data is loaded.
comparison of structures of Geobacillus stearothermophilus and Escherichia coli YiiM proteins. Both consist of a beta-barrel and two alpha-helix bundles and feature a cavity surrounded by the three modules. The cavity is characterized by positive electrostatic potentials and high sequence conservation. In silico docking of molybdenum cofactor
-
comparison of structures of Geobacillus stearothermophilus and Escherichia coli YiiM proteins. Both consist of a beta-barrel and two alpha-helix bundles and feature a cavity surrounded by the three modules. The cavity is characterized by positive electrostatic potentials and high sequence conservation
structure of the fusion protein comprising T4 lysozyme and N-terminally truncated human mARC1, to 1.78 A resolution. Residue D209 seems to have a Moco-independent impact on mARC enzymatic activity. F237 is the central amino acid of a hydrophobic core between the large beta-barrel and helices alpha4, alpha7, and alpha8, securing the 3D arrangement of the Moco binding site

 results (
results ( results (
results ( top
top





